Plan to incorporate pregnant ladies in drug trials ‘a generational change’ | EUROtoday
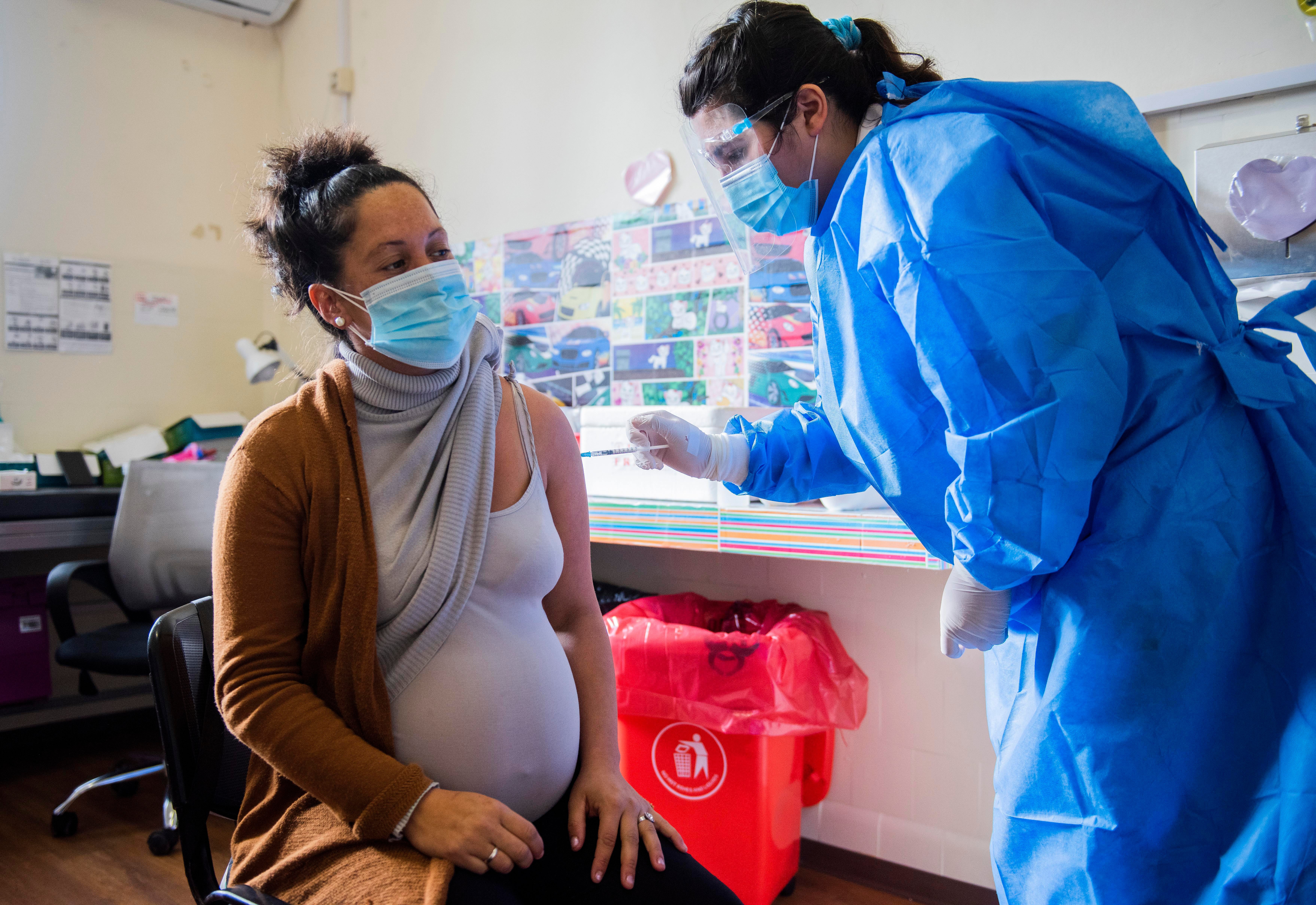
When Emma* determined to strive for a child, she started to come back off a few of the medicines she relied on to handle her Ehlers-Danlos syndrome. The complicated situation affecting connective tissues has left the 35-year-old with no bladder and being fed through a tube into her small bowel. But there have been some medication she couldn’t safely go with out. That’s when Emma realised nobody may inform her for positive whether or not these medication may hurt her child.
“The vast majority of the information that’s available is like, ‘to be used if there’s no other options, no research done’. And without the medication I will end up in hospital so I don’t really have an option but to take it,” Emma says. The lack of know-how left her feeling “guilt and anxiety”.
More than 90 per cent of medicines have by no means been examined in being pregnant, leaving hundreds of thousands of girls around the globe making this inconceivable selection: go with out remedy or take it with out full-throated reassurance from docs that it’s protected. This 12 months, within the greatest step change in a technology – because the Thalidomide scandal of the Fifties and Nineteen Sixties – the World Health Organization (WHO) will start to work with scientists, docs and drug builders to vary this.
That tragedy is the place the origins of this example lie. In the Fifties, a drug that had by no means been examined in pregnant ladies was given as a remedy for morning illness. At the time, scientists didn’t know that medication may move from mom to foetus by way of the placenta. It took years to grasp that it was inflicting severe delivery defects, together with shortened or lacking limbs.
The Thalidomide scandal led to the creation of the drug security monitoring system that also exists at present within the UK, in addition to the passing of the Medicines Act 1968. This regulation set out strict guidelines round how medicines needed to be examined earlier than they could possibly be given to sufferers. But pregnant ladies – whose experiences led to the creation of those security nets – have been systematically excluded from trials. Not out of malice however out of concern, explains Mariana Widmer, a maternal well being scientist at WHO.
“People have been scared to treat pregnant women since the thalidomide tragedy,” Widmer says, and “pregnant women are scared to be treated”.
“There’s nobody single organisation or one particular person that may make this modification. This change is big. This take time,” she provides. “We need collaboration and we need partnerships. And this is what we at WHO would like to do…bring together all these players at the table and work together to make this change, that’s the only way to do it”.
While the spectre of inflicting life-long disabilities in infants led to an perspective that it was higher to not take the danger in any respect, what was uncared for was the danger of leaving pregnant individuals out. They may have diseases that want remedy like another a part of the inhabitants. At the second, it’s usually left to midwives and docs to have a troublesome dialog with them – that they may want drugs to maintain them and their infants nicely, however they, “need to know that this medicine has never been tested in pregnant women,” Widmer says, that means we are able to’t make sure of their results.
After lethal bleeding, the subsequent most typical class of maternal deaths around the globe is from continual diseases together with coronary heart situations, diabetes and epilepsy. “Pregnant women are still dying from conditions that are otherwise preventable in others,” she says.
Emma is all too conscious of this “catch-22”. One of her drugs, Nabilone, is an artificial model of the compound THC present in hashish. She takes it for relentless nausea and vomiting.
“Before I had the [Nabilone]I was in hospital every few weeks needing IV replacements and electrolyte replacements. So it’s all being deemed safer that I stay on them because we know what’s going to happen if I don’t. And that’s probably more dangerous”.
Because of a scarcity of randomised trials, a lot of the proof on THC-use throughout being pregnant comes from leisure hashish customers who’re additionally extra prone to smoke cigarettes and drink alcohol – recognized dangers to a foetus – making the information “meaningless”, Emma was informed.
Similarly, coming off opiate painkillers may enable her ache to spiral to ranges that would put her vulnerable to shedding the being pregnant, she was informed. “It’s this constant weighing up of what is more dangerous, what’s not more dangerous and it’s really, really difficult to make that judgement,” she says.
The WHO crew plans to work by way of an inventory of important medicines for the commonest continual ailments. They will produce data for individuals testing medication, guiding them on the way to embody pregnant ladies safely. The subsequent step is to incentivise drug builders to prioritise this work, partly by speaking to regulators about fast-tracking the medicines as soon as examined.
We aren’t in the identical place as we have been some 70 years in the past when Thalidomide began to be provided for morning illness, Widmer explains. Scientists have a a lot better understanding of the way to safely and totally check medication, and trials are carefully regulated.
“We have the means to know how to do it properly in order to ensure that the woman is safe and that the baby is safe,” she says.
That entails testing medication first in cells in petri dish and in animals, then in wholesome non-pregnant individuals, adopted by non-pregnant individuals with well being situations. “It’s step by step. It’s not that we will go directly to pregnant women to be included in clinical trials,” Widmer says.
Drug trials are notably managed and carefully monitored environments, making it simpler to choose up any issues than it’s within the normal inhabitants.
Dr Teesta Dey labored delivering infants within the NHS for a decade and now consults on international well being, nevertheless it was changing into a brand new mum herself that resolved her to do that work.
While going by way of an advanced being pregnant along with her now 15-month-old, she was taken abruptly by how troublesome it was to know what medicine she may and couldn’t take. Labels have been unclear or contradictory. “That left me in a real quandary because I didn’t know, could I use it? Is it safe for me? Is it safe for my baby? And that put a huge burden on me to make that decision as a mother.”
When Dr Dey lately spoke at a Birth Trauma Association convention chaired by ladies’s well being minister, Baroness Gillian Merron, she heard numerous tales from ladies who had been left not realizing whether or not they may take remedies for every thing from excessive being pregnant illness to coronary heart situations. “So the reality of what happens is that people will take it off-label,” which “puts that onus on the women making that choice” whereas well being suppliers are compelled to say “I don’t know”.
“We’re extrapolating it from male [and non-pregnant] models that have shown that it’s safe and effective,” she says. “But we know that the pregnancy state is very different. Even your circulating blood volume is increased by 40 per cent”. Testing a medication on all teams who would possibly use it, however, would give us extra dependable details about the way to safely deal with individuals.
“I think sometimes we focus on the risk a lot…but we don’t ever think about what are the benefits here,” Dr Dey provides. “Shifting our mindset is really important.”
A very good instance, she says, is what occurred throughout the Covid-19 pandemic, when 75 per cent of vaccine research didn’t embody pregnant and lactating ladies.
“We also know from the data that this was the population of women who had the poorest outcomes, who were in hospital the most, who were burdened with this disease the most. Yet they didn’t include them in the study,” Dr Dey says. Because so many individuals acquired the vaccine in a brief area of time – together with those that could not have recognized they have been pregnant – it quickly grew to become clear that the vaccine was certainly protected. But the shortage of early data sowed distrust.
“It’s not that I want all pregnant women regardless of the condition to participate,” Widmer stresses, however that the potential results on pregnant ladies needs to be thought of from “point zero” when somebody decides to develop a drug that would profit a girl throughout her being pregnant.
A sea-change like this has occurred earlier than, in medicines for youngsters. Until roughly 20 years in the past, youngsters have been typically excluded from trials and in the event that they wanted to be given medicines, they have been handled as small adults for the needs of dosing – regardless of being physiologically completely different.
In 2007, a regulation handed by the European Union mentioned that if drug firms needed their merchandise to be licensed for youngsters, they have to check them on youngsters. Now, it’s routine that that is carried out the place protected. “This is something that I would love to do with pregnancy,” Widmer says.
A WHO toolkit of knowledge for scientists and sufferers is ready to be launched within the Spring.
It’s not simply a problem of security, Dr Dey believes, however one among selection. She is important of the “paternalistic model that has fallen where women are actively excluded without even being given the opportunity to participate in a trial”.
Pregnant ladies are able to understanding the dangers and making the choice whether or not to participate in a trial for themselves, she says – one thing Emma says she would contemplate if given the prospect.
Instead, Dr Dey says, “we’ve taken that autonomy and that control away”.
*Name has been modified
This article has been produced as a part of The Independent’s Rethinking Global Aid undertaking
https://www.independent.co.uk/news/health/pregnancy-drug-trials-women-who-b2898829.html
